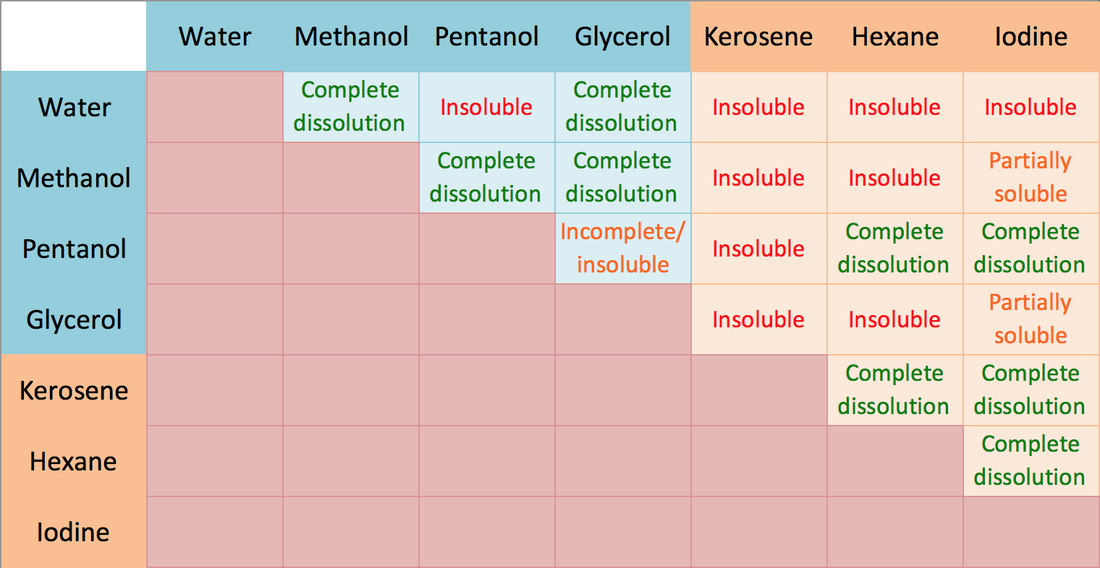
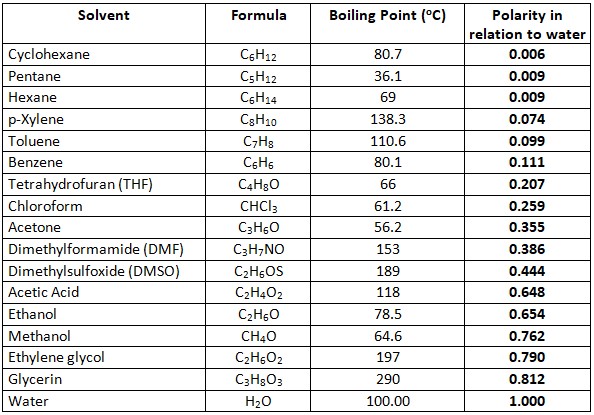
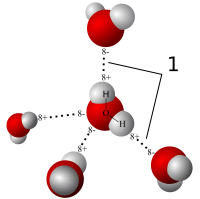
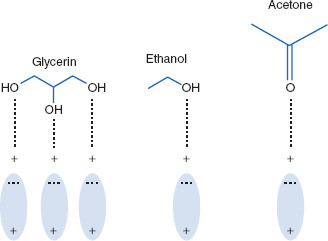
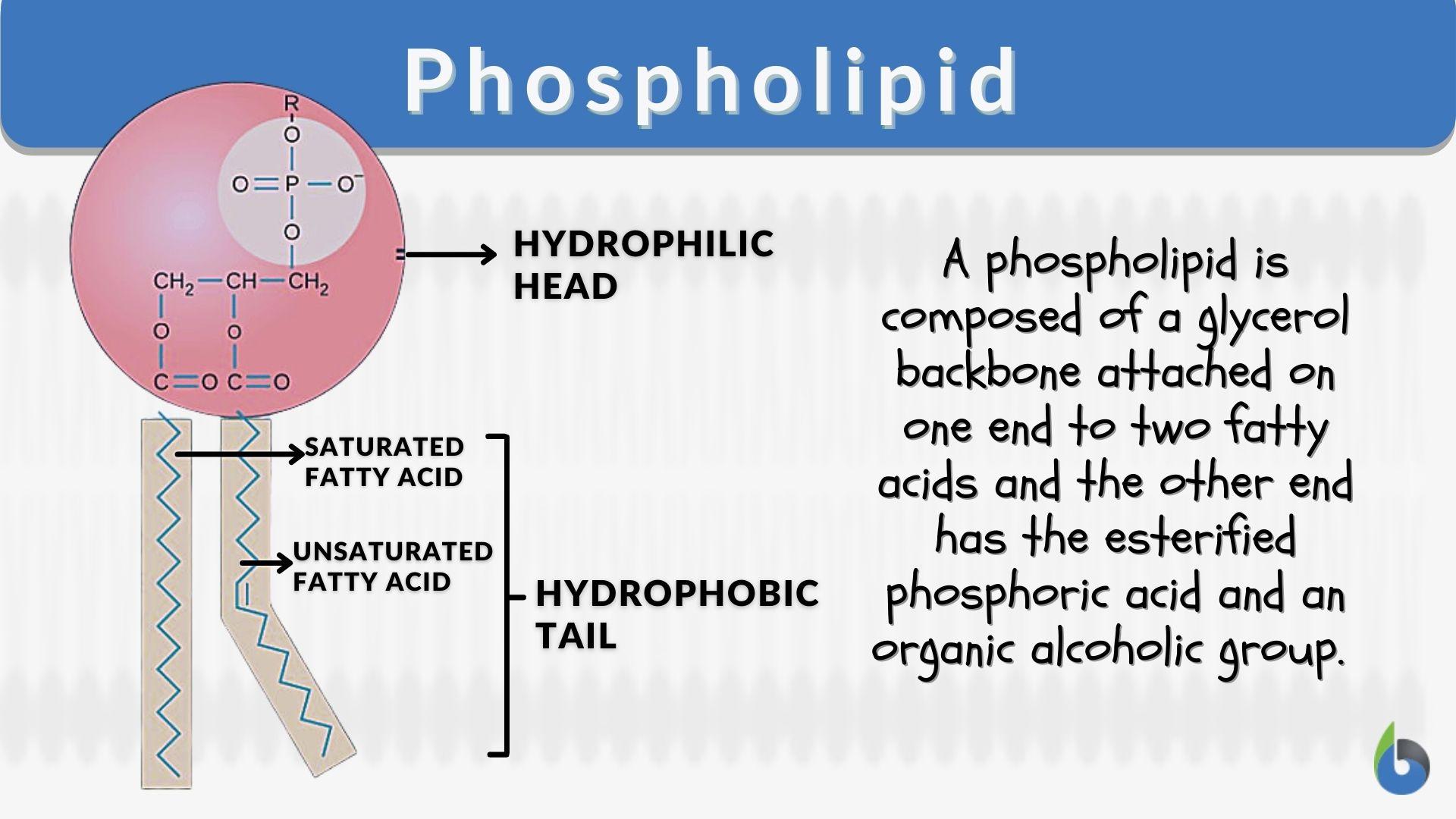
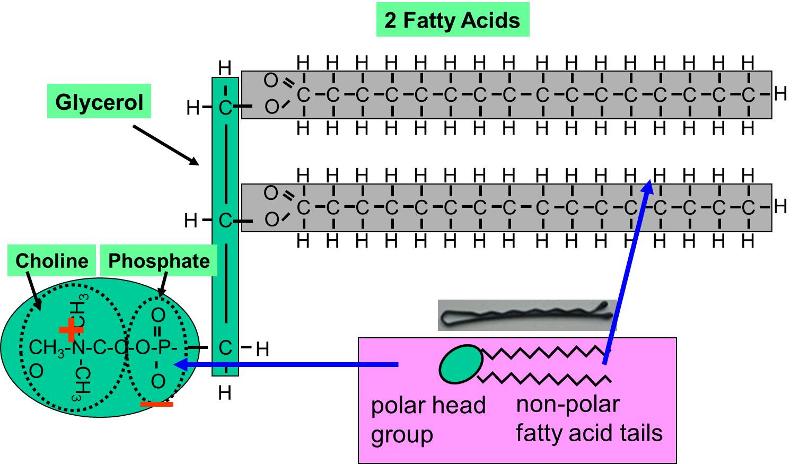
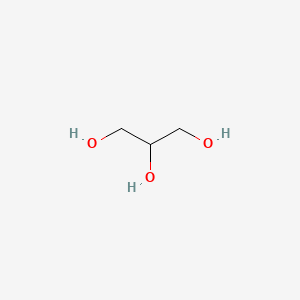
Lipids. Lipids are non-polar (hydrophobic) compounds, soluble in organic solvents. 1. Simple lipids: esters of FA with alcohols Fats: alcohol = glycerol. - ppt download
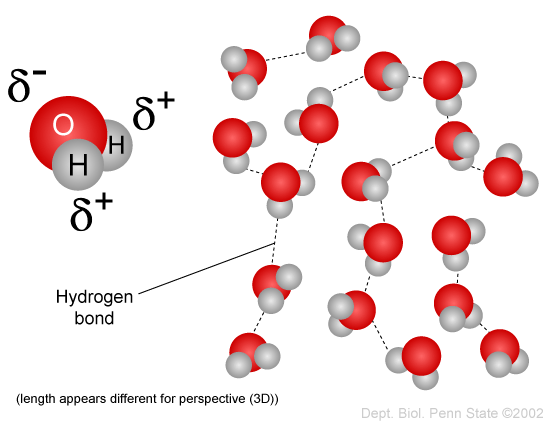
Since the head of the phospholipid contains both glycerol (non-polar) and phosphate (polar) group, why is the head considered polar overall? - Quora

Lipids & Membranes. Lipids Lipids are non-polar (hydrophobic) compounds, soluble in organic solvents. Most membrane lipids are amphipathic, having a non-polar. - ppt download
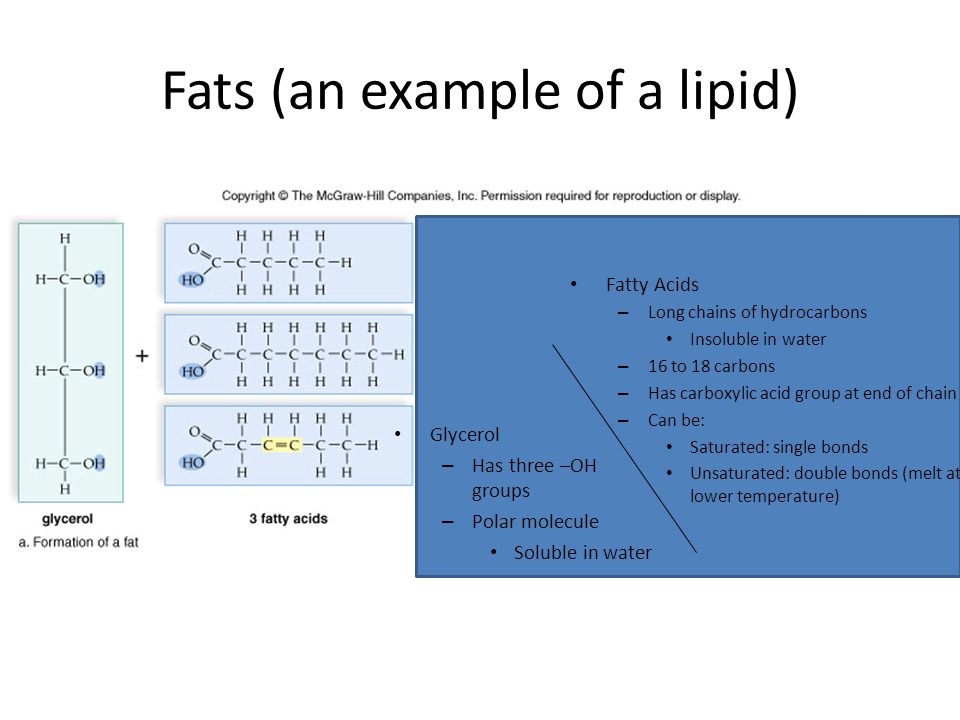
Fats (an example of a lipid) Glycerol – Has three –OH groups – Polar molecule Soluble in water Fatty Acids – Long chains of hydrocarbons Insoluble in water. - ppt download